
SGN-CD30C, an investigational CD30-directed ADC, utilizes a potent novel camptothecin derivative as the cytotoxic payload. SGN-CD30C targets the same antigen as brentuximab vedotin (BV), though the payload has a different mechanism of action, namely inhibiting topoisomerase I rather than disrupting microtubules. Unlike monomethyl auristatin E, camptothecin-based therapies do not cause peripheral neuropathy clinically, suggesting that SGN-CD30C may have the potential to avoid one of the most common adverse events associated with BV.
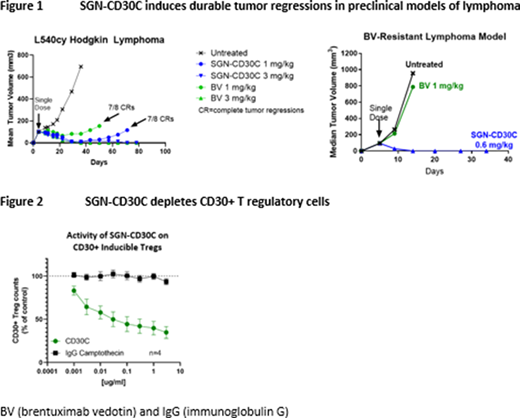
In preclinical studies, SGN-CD30C demonstrated strong monotherapy activity, inducing durable tumor regressions in multiple lymphoma models and eliciting cures in a BV-resistance tumor model (Figure 1). Moreover, SGN-CD30C exhibits many of the desirable attributes associated with BV, notably, bystander activity and CD30+ T regulatory cell (Treg) depletion. Using an admixed model of CD30+ and CD30-tumor cells, SGN-CD30C demonstrated robust anti-tumor activity in vivo, confirming SGN-CD30C can induce bystander killing of antigen-negative tumor cells in CD30-heterogeneous tumors. Additionally, SGN-CD30C depleted CD30+ Treg cells in vitro (Figure 2), suggesting it has the potential to exert activity through multiple mechanisms of action.
SGN-CD30C was well-tolerated in non-human primate toxicology studies and demonstrated ~6-fold higher maximum tolerated dose when compared to BV. The primary toxicity for SGN-CD30C in non-human primates (NHP) studies was anemia due primarily to bone marrow suppression of erythropoiesis. SGN-CD30C had no effect on neutrophil counts and caused only minimal to mild decreases in platelets. The lack of significant neutropenia seen with SGN-CD30C contrasts with BV, indicating the two ADCs may have non-overlapping dose limiting toxicities. Hematology parameters show SGN-CD30C is tolerated at a 10-fold higher dose than BV with weekly dosing, suggesting SGN-CD30C may offer the potential for increased dose density.
In summary, our data show SGN-CD30C is a compelling therapeutic candidate for CD30-positive malignancies. The distinct mechanism of action, strong anti-tumor activity, and enhanced tolerability provide a strong rationale to clinically investigate SGN-CD30C across the CD30-expressing lymphoma landscape. A Phase 1 clinical trial is planned to investigate SGN-CD30C in relapsed and refractory lymphoma.
Heiser:Seattle Genetics: Current Employment, Current equity holder in publicly-traded company. Grogan:Seattle Genetics: Current Employment, Current equity holder in publicly-traded company. Jin:Seattle Genetics: Current Employment, Current equity holder in publicly-traded company. Conerly:Seattle Genetics: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal